Wisconsinites Who Use Medical Marijuana Wait For Laws And Research To Catch Up
While a variety of people have found success treating their medical ailments with cannabis, the drug remains illegal in Wisconsin.
November 11, 2019

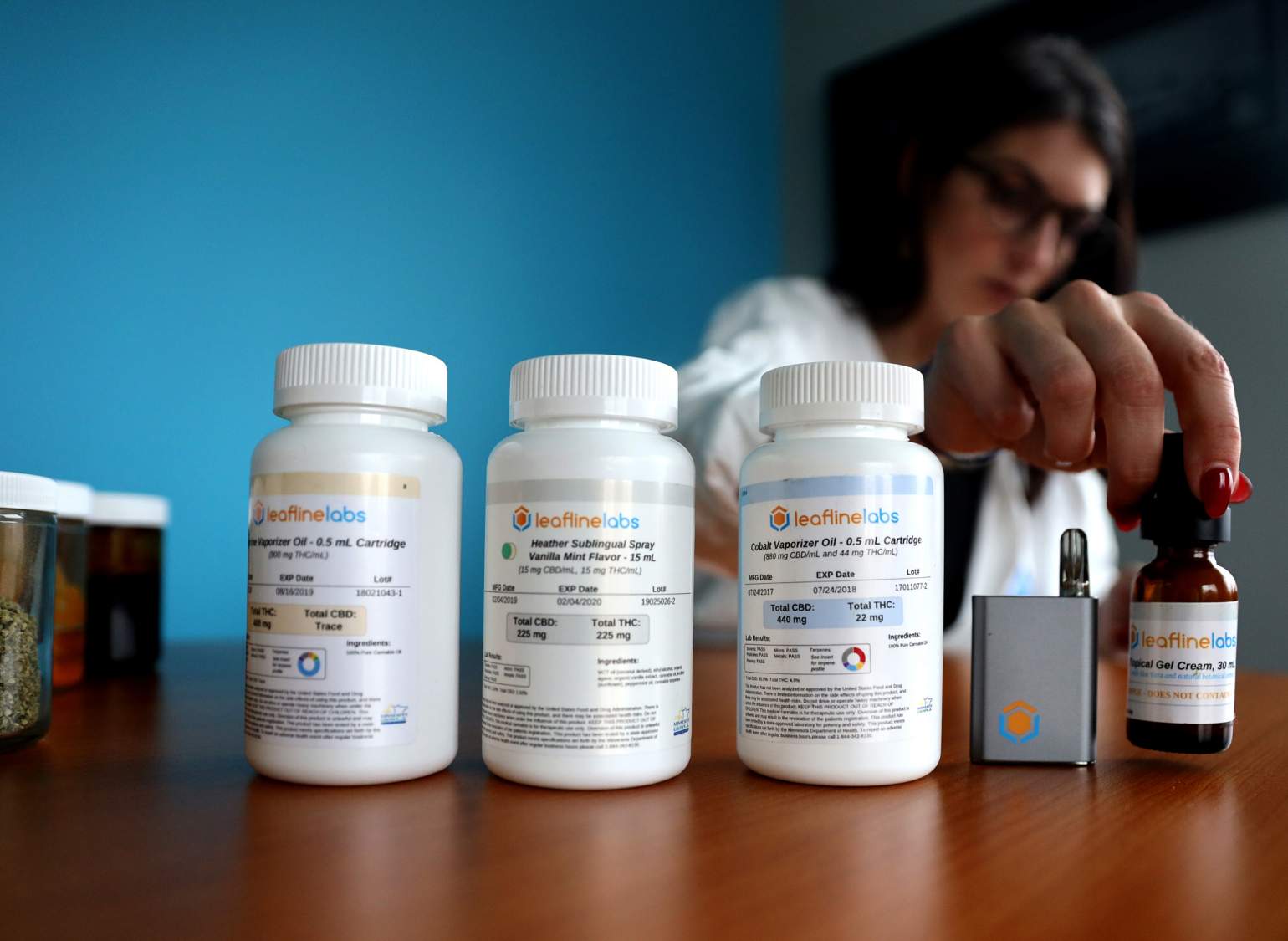
Leafline Labs cannabis oil poured into syringe

After four decades of using strong prescription drugs to treat Crohn’s disease, a chronic digestive disorder, Patty developed an aggressive form of skin cancer.
“It’s because my body has been suppressed for so long, it can’t fight it [cancer],” the Wisconsin resident said.
Patty, who has worked at her father’s restaurant for 27 years, now struggles to handle full-time duties.
“I’m trying to get disability, but I’ve been denied once already. I don’t plan on quitting working. I just need help. I need help because I can’t do a full-time job,” Patty said.
In March 2017, a friend who lives in New Mexico, where medical marijuana is legal, mailed her Buddha Tears, a cannabis oil product containing cannabidiol, or CBD, and delta-9-tetrahydrocannabinol, or THC, the psychotropic component of cannabis. After consuming a tiny amount of the oil each day — as well as smoking marijuana — Patty said she saw a massive improvement in her condition.
“Unfortunately, I have to smoke everyday, because if I don’t, I will be in the bathroom all the time,” said Patty, who asked that her last name not be published because she is using an illegal substance.
But these days, Patty is again struggling with the symptoms.
“My connection [for CBD and marijuana] got cut off,” she said. “I’m very angry.”
While Patty and others have found success treating their medical ailments with cannabis, the drug remains illegal in Wisconsin.
And because of its status as a Schedule I drug — the most restrictive classification — there has been limited research in the United States about its effectiveness as medicine. The U.S. Food and Drug Administration has authorized one component of cannabis to treat serious and rare seizure disorders, as well as three drugs with synthetic cannabis substances; no other uses have been approved.
Although it remains illegal federally, 33 states and the District of Columbia have authorized medical use of cannabis. A bipartisan group of lawmakers has proposed legalizing it for medical use in Wisconsin, and another group of Democratic lawmakers introduced a bill in October 2019 to decriminalize possession of less than 28 grams. But Senate Majority Leader Scott Fitzgerald, R-Juneau, remains opposed.
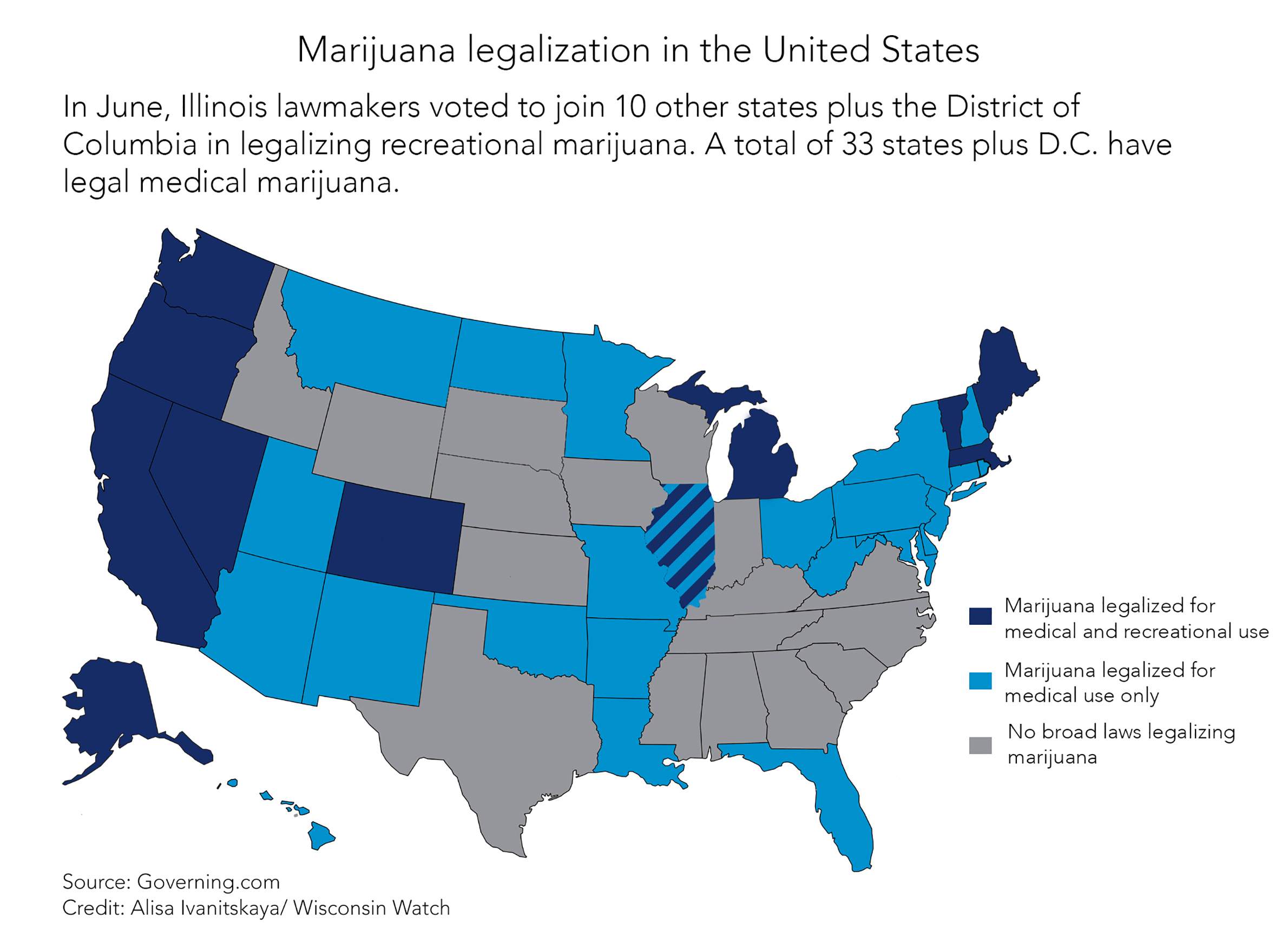
An April poll conducted by the Marquette Law School Poll showed that 83% of registered voters polled support the use of marijuana for medical purposes with a doctor’s prescription.
“When issues receive more than 70% support from registered voters in Wisconsin, the Legislature needs to listen and act,” said Rep. David Bowen, D-Milwaukee.
Marijuana misclassified?
According to Dr. Angela Janis, director of psychiatry for University of Wisconsin-Madison’s University Health Services, Schedule I drugs, including marijuana, are considered to have no currently accepted medical use and a high potential for abuse, whereas for Schedule II drugs, there is less potential for abuse, and there is some therapeutic benefit.
Janis is intimately familiar with this distinction. In addition to her university job, Janis is chief medical officer at LeafLine Labs, a Minnesota-based medical marijuana company.
“To put this in perspective: Methamphetamine is Schedule II because it’s approved for obesity. Cocaine is Schedule II because it’s approved for nasal surgery since it can constrict your blood vessels as they do surgery in your nose. So that’s the bar for what ‘medical benefit’ means,” Janis said.

According to Janis, cannabis has less abuse potential than any of those substances.
“Cannabis is not appropriately scheduled. And that’s one of the barriers, but not the only barrier, to research,” Janis said.
Janis recommends rescheduling the drug so researchers can further study its properties. Even Smart Approaches to Marijuana, which opposes marijuana legalization, is “fully supportive” of drugs containing cannabis that have been approved by the FDA, said Colton Grace, a spokesman for the group.
How marijuana works in the body
According to the National Institute on Drug Abuse, cannabinoids are substances within the cannabis plant that act on specific receptors in the human brain and body. They are the main active ingredients in the medical products derived from cannabis.
These receptors affect many essential functions, including one’s memory, thinking, concentration and coordination. Interfering with it can have profound effects — both positive and negative.
Two of the most extensively studied cannabinoids are THC and CBD. However, there are dozens of cannabinoids that may also have medical uses.
“Many strains of the cannabis plant can have 60, 70, 80 cannabinoids in them that all interact in different ways,” Angela Janis said.
The National Institutes of Health reported spending $191 million on researching cannabinoids for medicinal use in 2017-18.
Some effects are already known. For example, THC can affect the central nervous system, producing benefits such as decreased vomiting and nausea, increased appetite, reduced pain and anti-inflammatory effects. CBD also acts as an anti-inflammatory, increasing immune function, reducing pain and keeping certain cells from proliferating.
Cannabinoid receptors are not in areas that control breathing, which is why there are no fatal overdoses with marijuana, Janis said. CBD actually blocks the psychotropic effects of THC, Janis said.
In addition to all those cannabinoids, the Cannabis sativa plant has a lot of other chemicals. For instance, terpenes, which give each strain its particular smell, such as lemon or pine, “are thought to have a lot of effects, but we just don’t know what they actually do in the body,” Janis said.
An effective treatment for pain
In 2017, the National Academies of Sciences, Engineering and Medicine came out with one of the most comprehensive reviews of scientific research on what is known about the health effects of cannabis and cannabis-derived products. The committee considered more than 10,000 scientific abstracts. It reached nearly 100 conclusions, finding substantial evidence for just a few indications — the biggest one being pain.
The report found there is substantial evidence that cannabis is an effective treatment for chronic pain in adults, specifically nerve pain, Janis said.
The group also found conclusive evidence for cannabis treating chemotherapy-associated nausea and vomiting and multiple sclerosis-associated muscle spasms.
The report also showed moderate evidence that cannabis or cannabinoids are effective for improving sleep in individuals with sleep apnea, fibromyalgia, chronic pain and MS.
It also found limited evidence for cannabis as effective for increasing appetite and decreasing weight loss associated with HIV/AIDS, relaxing muscle tightness and pain from MS, symptoms of Tourette syndrome, anxiety and post-traumatic stress disorder.
Anecdotal evidence has also proven the effectiveness of cannabinoids for treating Rett syndrome.
Norah Lowe, 10, started feeling relief from the rare neurological disorder one year ago when she began using CBD to treat her symptoms. Rett syndrome impacts nearly every part of a child’s life, including the ability to speak, walk, eat and breathe. A distinct feature of the condition is repetitive, almost constant hand movements.
Norah, who uses a wheelchair, has experienced “increased flexibility, decreased pain and muscle cramping, increased communication, cognitive ability, reduction in seizures, better mood control, and the list goes on and on,” her father, Josh Lowe, said.
At a news conference arranged by state Rep. Melissa Sargent, D-Madison, to introduce her latest bill to legalize medical and recreational marijuana, Lowe said he is frustrated that state law prohibits Norah from trying medical marijuana, which has helped others with her condition.
A 2017 study published in the Cochrane Database of Systematic Reviews analyzed several studies, concluding that cannabis-based medicines were better than placebos for pain relief — and that these medications also improved sleep and psychological distress — it concluded that any potential benefits might be outweighed by their potential harms.
According to the Marijuana Policy Project, the most common conditions accepted by states that allow medicinal cannabis relate to the relief of the symptoms of cancer, glaucoma, HIV/AIDS and MS. Some other common indicators include Alzheimer’s disease, inflammatory bowel disease, Crohn’s, Parkinson’s disease and PTSD, according to the group, which advocates for marijuana legalization.

Additionally, the University of Michigan published a study in the February 2019 issue of Health Affairs to understand the reasons why people are using cannabis for medical purposes, and whether those purposes are evidence-based.
The authors found that 85.5% of uses of medical cannabis were for conditions for which there was substantial or conclusive evidence of their therapeutic effectiveness. Even more, they found that chronic pain is currently the most common qualifying condition reported by medical cannabis patients, used by 64.9% of such patients in 2016.
“That’s a good sign,” Janis said. “Even though a physician can write it [a cannabis prescription] for a variety of things, it seems to be being used for what it’s intended for.”
Barriers to research continue
Since cannabis is a Schedule I drug, it is “very difficult to study at any institutional level” because, in order to do so, researchers need sign-offs from the U.S. Drug Enforcement Agency, which has “historically been unwilling to provide them,” said David Abernathy, vice president of data and government affairs for the Arcview Group, a firm that advises investors in the cannabis industry.
Because of this, “things like double-blind placebo-controlled clinical trials weren’t happening in the U.S.,” Abernathy said. But there has been a lot of research in the past decade in other countries including Israel, Canada, China and Italy, and “now we’re starting to see more research in the U.S.,” he said.
The 2017 National Academies review of cannabis research agreed that the drug’s status as a Schedule I substance made it hard to study.
“Researchers also often find it difficult to gain access to the quantity, quality, and type of cannabis product necessary to address specific research questions,” the review found.
Patty, the Crohn’s patient, believes that her cannabis treatment not only alleviated her Crohn’s symptoms, but she credits it with keeping her aggressive skin cancer at bay.

According to a 2018 article published in Biochemical Pharmacology, studies have shown the potential of cannabinoids to reduce of skin cancer progression. However, there is a significant lack of clinical studies promising enough to make any conclusive statements at this time.
“I haven’t had the cannabis oil since March of 2018, and once I couldn’t get it anymore, I mean I just finished my 12th surgery [for cancer],” Patty said. “So, you tell me, what do you think?”
This story was produced as part of an investigative reporting class at the University of Wisconsin-Madison School of Journalism and Mass Communication under the direction of Dee J. Hall, managing editor of Wisconsin Watch. The Cannabis Question is a series exploring questions about proposals to legalize marijuana in Wisconsin. Wisconsin Watch’s collaborations with journalism students are funded in part by the Ira and Ineva Reilly Baldwin Wisconsin Idea Endowment at UW-Madison. The nonprofit Wisconsin Watch collaborates with Wisconsin Public Radio, Wisconsin Public Television and other news media. All works created, published, posted or disseminated by Wisconsin Watch do not necessarily reflect the views or opinions of UW-Madison or any of its affiliates.
 Passport
Passport















Follow Us